FIT + mt-sDNA - Cologuard

More Sensitive than FIT Alone
Cologuard combines DNA testing and stool blood detection, offering enhanced sensitivity compared to FIT (Fecal Immunochemical Test) in identifying colorectal cancer and advanced precancerous polyps.

At Home Convenience
The test is non-invasive and can be completed in the comfort of your own home, with no dietary restrictions or preparation required. Colonoscopy is required if mt-sDNA is posivite.

More Sensitive than FIT Alone
Cologuard combines DNA testing and stool blood detection, offering enhanced sensitivity compared to FIT (Fecal Immunochemical Test) in identifying colorectal cancer and advanced precancerous polyps.
Test Process and Next Steps
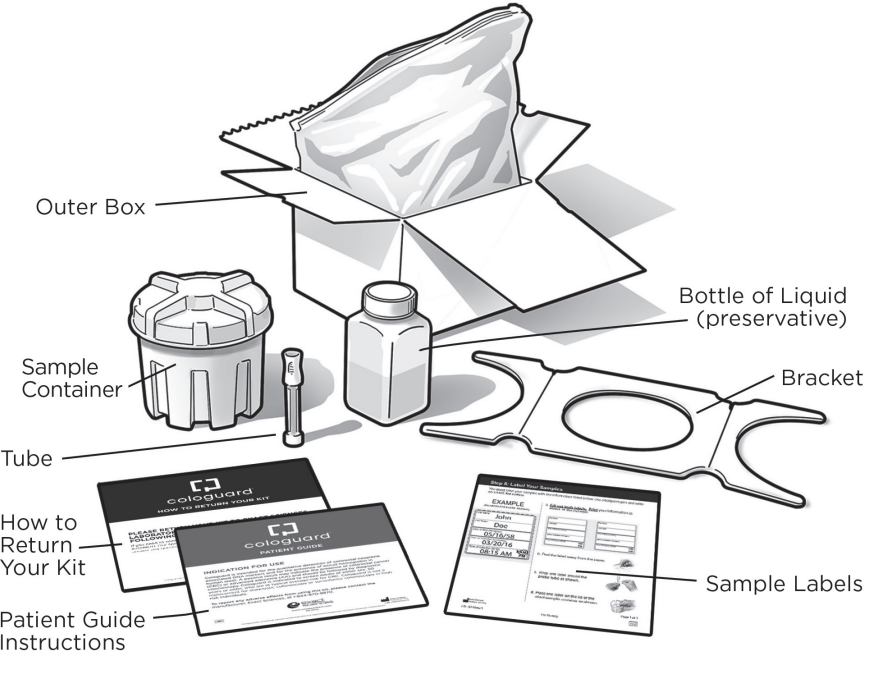
Kit Ordered by Your Doctor and Mailed to Your Home
Your test kit will have instructions to guide you through the testing process. There are no preparation or dietary requirements prior to completing the stool sample collection.
Sample Collection
Place the included bucket under your toilet seat to collect your stool. After your bowel movement, swab a small sample from the stool for the FIT portion of the test. The entire sample is sealed carefully and sent in the prepaid box for lab analysis within 72 hours of collection.


Test Results and Next Steps
Test results are typically available in less than two weeks. A positive test indicates blood and/or abnormal DNA detected in the sample and a follow-up colonoscopy is required to complete the screening process. For negative results, current guidelines recommend repeating the test in three years to maintain regular screening and ensure continued monitoring.
Risks and Limitations of FIT + mt-sDNA Testing
While the sensitivity of Cologuard is improved compared to FIT, it still has limitations. This test misses approximately 8% of colorectal cancers and is less reliable at detecting precancerous lesions, including those with high-grade dysplasia, the stage just before a polyp turns into cancer.
Test completion requires follow-through with a colonoscopy in the event of a positive result, yet currently, just over 50% of patients with a positive non-invasive test proceed to a colonoscopy within a year.
- Detects 92% of colorectal cancer
- Detects 69% of polyps with high grade dysplasia
- Detects 42% of all advanced precancerous lesions (APL)


